The Pharma Manufacturing World Summit is a premier gathering of industry leaders, pharmaceutical manufacturing executives, and experts dedicated to exploring innovative strategies, emerging trends, and best practices in the dynamic world of pharmaceutical management.
PMWS gathers SVPs, VPs, and Directors of Manufacturing, Technical Operations, Quality, and Supply Chain, Technology Leaders, and Plant/Site/Facility Managers to share insights, foster innovation, and build invaluable connections. Dive into a thoughtfully curated environment that brings together thought leaders, allowing you to explore new ideas and engage in meaningful discussions. Join the conversation at PMWS and be part of a community dedicated to pushing the limits of pharmaceutical manufacturing success.







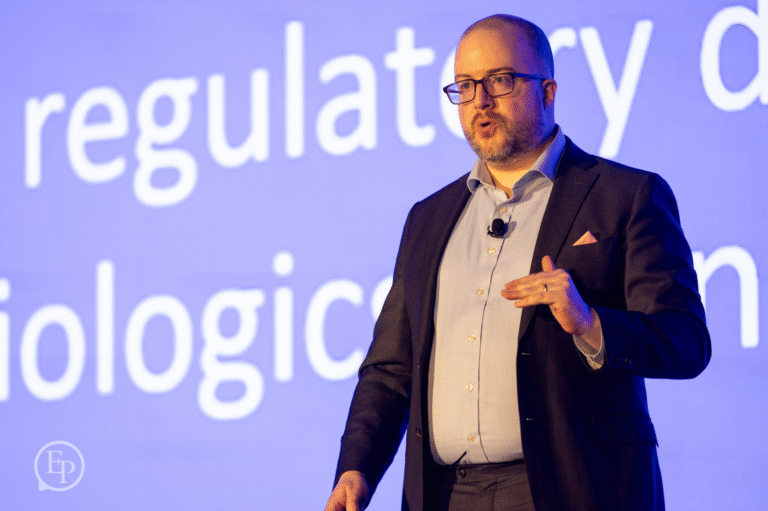
Year after year, we bring to the stage today's thought leaders and innovators

Eamonn Warren serves as Group Vice President of API and Dry Products Manufacturing at Eli Lilly and Company, where he leads the global network of internal and external manufacturing sites responsible for active pharmaceutical ingredients and dry-product production. He assumed this role in 2024 and is a member of Lilly’s Manufacturing Leadership Team.
Eamonn began his career with Lilly in 1995 at the Kinsale manufacturing site in Ireland as an automation engineer. Over the course of nearly three decades, he has held roles of increasing responsibility across engineering, operations, Six Sigma, human resources, and site leadership. His international experience includes assignments in Puerto Rico, Indianapolis, Augusta in the state of Georgia, and the Fegersheim manufacturing site in France.
Prior to his current position, Eamonn served as the global lead for Lilly’s Parenteral Manufacturing Network, where he oversaw strategy, performance, and modernization across Lilly’s sterile manufacturing capabilities. He is recognized for championing advanced and innovative manufacturing approaches as well as for his focus on operational excellence, talent development, and regulatory rigor.
A native of Ireland, Eamonn holds a degree in Chemical Engineering from University College Dublin and a degree in Management and Marketing from University College Cork.
Eamonn Warren
Group Vice President, Global API & Dry Products Manufacturing
Eli Lilly


Dr Mire-Sluis is currently Head of Quality for Gilead Sciences. He was Head of Global Quality for AstraZeneca and Vice President, North America, Singapore, Contract and Product Quality at Amgen Inc. He was previously Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, FDA, in Bethesda, Maryland.
He trained in Genetics and Biometry and has a PhD in Cell biology and Biochemistry. Dr Mire-Sluis was the Head of the Cytokine Group in the Division of Immunobiology at the National Institute for Biological Standards and Control, Potters Bar, UK. Dr Mire-Sluis specialized in the development of assays for the characterization and quantitation of biological products. He then became Director of BioAnalytical Sciences at Genentech and prior to joining FDA, was Executive Director of Analytical Sciences at CancerVax Corporation, San Diego.
He is the Chairman of the IABS Biotherapeutics Committee, Vice Chairman of the USP Biologicals Characterization Expert Committee, an expert for the International Committee for Harmonization and on the board of the Journal of Immunological Methods
Anthony Mire-Sluis
Senior Vice President, Global Quality
Gilead Sciences


Glenn A. Gerecke is responsible for manufacturing, supply chain and distribution around the world. He joined Sandoz in 2022, having previously held senior operational roles at Phlow Corporation, Teva Pharmaceuticals and Bristol Myers Squibb. Glenn A. Gerecke has led shop floor and manufacturing support teams, multiple-technology manufacturing sites, regional manufacturing operations, as well as global engineering/facilities and human resources organizations for more than 35 years. He holds a bachelor of science degree in chemical engineering from Worcester Polytechnic Institute, as well as master’s degrees in business and management from the University of Massachusetts and Worcester Polytechnic Institute, respectively, and a doctorate in business from Capella University.
Glenn A. Gerecke
Chief Manufacturing and Supply Officer
Sandoz


Joanne is a seasoned biotech executive with over 30 years of experience across large pharma and early-stage biotech in the areas of operations, leadership, product development, process development, clinical & commercial manufacturing, and supply chain. Over the course of her career, she has contributed to the licensure and commercialization of numerous medicines across different modalities and therapeutic areas. She has fostered high-performing, empowered individuals and teams and mutually beneficial partnerships with both internal and external stakeholders.
Before Elektrofi, Joanne most recently served as the Chief Technical Officer at Abata Therapeutics, a company developing Treg cell therapies for serious autoimmune and inflammatory diseases. Prior to Abata, she served as the Chief Technical Officer at Aerium Therapeutics, Inc. and as the Chief Operating Officer and interim CEO at Boston Pharmaceuticals. Previously, Joanne served as the Executive Vice President of Global Pharmaceutical Development and Operations at Celgene Corporation where she oversaw the company’s Product Development, Global Manufacturing Operations, Supply Chain, Engineering and Quality functions. Joanne also served in leadership positions at other pharmaceutical and biotech companies, including Shire, Abbott Laboratories, Amgen, and Genentech. She has served as a member of the Board of Directors of public biopharmaceutical companies, including Orchard Therapeutics and Astria Therapeutics.
Joanne holds a Ph.D. in Biochemistry and Molecular Biology from Oregon Health and Science University and completed a postdoctoral fellowship in the department of Pharmaceutical Chemistry at the University of California, San Francisco.
Joanne Beck
Chief Operating Officer
Elektrofi
The Pharma Manufacturing World Summit 2026 is designed to ensure every attendee walks away with real & measurable strategies to bring back to their organizations.
Director Quality Assurance, Eli Lilly and Company
Associate Director, Quality Management Systems, Bristol Myers Squibb
Director of QA, Merck Sharp & Dohme
VP Partnership and External Supply LM, Johnson & Johnson
Manager, QA System Management, Daiichi Sankyo
Vice President of Process Development, PTSI