MDMES gathers Chief Quality Officers, Chief Operations Officers, Chief Supply Chain Officers, EVPs, SVPs & VPs of Manufacturing, Quality, Medical Devices, Patient Safety, Supply Chain, Technical Operations Executives, and Regulatory and Compliance Executives to share insights, foster innovation, and build invaluable connections. Dive into a thoughtfully curated environment that brings together thought leaders, allowing you to explore new ideas and engage in meaningful discussions. Join the conversation at MDMES and be part of a community dedicated to pushing the limits of medical device success.
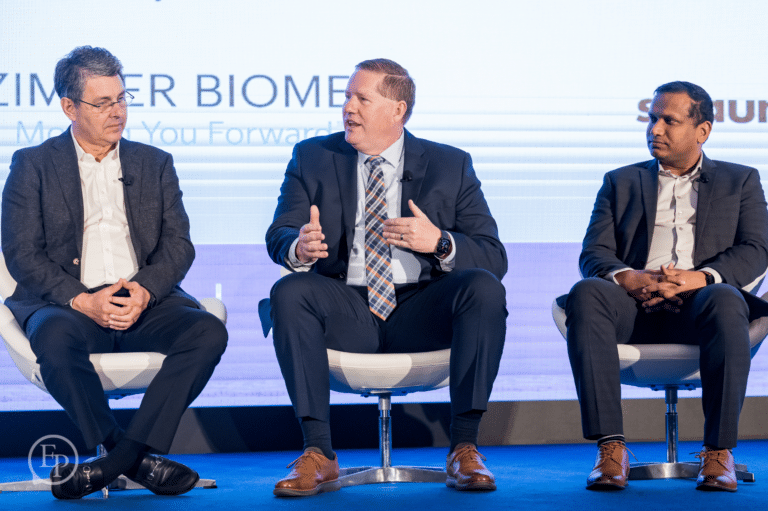




Year after year, we bring to the stage today's thought leaders and innovators

Dr. Michelle Tarver is a visionary public health executive, board-certified in ophthalmology with a doctorate in epidemiology, who serves as the Director of the Center for Devices and Radiological Health. She has spent more than 15 years as a medical device regulator, driving strategic initiatives, conducting clinical research, and changing organizational culture. Dr. Tarver has held various leadership positions while at FDA, including the Deputy Director of the Office of Strategic Partnerships and Technology Innovation and the Program Director of Patient Science and Engagement. Over the course of her career, she has conducted laboratory-based and epidemiological studies, clinical trials, and surveys to capture patient preferences, as well as developed registries and patient-reported outcome measures. Dr. Tarver has extensive policy experience in crafting regulations, guidances, and conducting premarket and postmarket reviews. She most recently served as the Deputy Center Director for Transformation, steering the development, implementation, and direction of CDRH’s transformative projects and strategic initiatives. Under her leadership, CDRH has launched efforts to amplify the perspectives of people living with medical conditions, foster collaboration across the healthcare ecosystem, and stimulate creative evidence generation pathways. Dr. Tarver received a B.S. in Biochemistry from Spelman College in Atlanta, GA and completed the M.D./Ph.D. program at The Johns Hopkins University School of Medicine and Bloomberg School of Public Health. As a dedicated clinician, she continues to care for people living with inflammatory eye conditions.
Michelle Tarver, M.D., Ph.D.
Center Director, Center for Devices and Radiological Health
U.S. Food and Drug Administration


In his role, Viju is responsible for global operations, including manufacturing, direct procurement, regulatory affairs, quality assurance, sustainability, integrated business planning, logistics and advanced operations such as additive manufacturing technology development.
Prior to joining us, Viju served as Chief Supply Chain Officer and Senior Vice President at Verizon. Before his work at Verizon, Viju held key leadership roles at Intel in technology, supply chain and manufacturing operations.
Viju earned his doctoral degree from the University of Pennsylvania and dual graduate degrees (MBA, S.M. in electrical engineering) from the Massachusetts Institute of Technology’s Leaders for Global Operations program. He also holds a graduate degree (M.S.E. in computer science engineering) from the University of Michigan and a bachelor’s degree in engineering from the University of Kerala, India.
Viju Menon
Group President, Global Quality & Operations
Stryker

Matt was recently promoted to Group Vice President, Supply Chain Operations for MedSurg and Neurotechnology (MSNT) for Stryker. He joined the company in 2023 as our VP or Global Manufacturing and previously served as Senior Vice President of Integrated Supply Chain for Becton Dickinson. With over 25 years of extensive manufacturing experience, Matt has a successful track record in medical device, pharmaceutical and industrial goods where he has elevated efficiency, improved supply chain resilience and enhanced collaboration. He earned his MBA in General Management from Harvard Business School and his bachelor’s degree in Manufacturing Engineering from BYU.
Matthew Smith
Group Vice President, Supply Chain Operations for MedSurg and Neurotechnology (MSNT)
Stryker


Mark P. Brosius is senior vice president and chief manufacturing and supply chain officer at Intuitive, with responsibility for SIOP, supply chain, new product introduction, manufacturing, and logistics operations. Brosius is a leading expert in enterprise scale manufacturing and business process automation, as well as the application of big data and analytics to planning, supply chain, and manufacturing. He brings more than 30 years of experience in multinational manufacturing operations and product development.
Brosius joined Intuitive in 2012 as the founding leader of the manufacturing automation function, before leading new product introduction (NPI) engineering and ultimately all of operations. He was promoted to his current role in 2023.
Prior to joining Intuitive, he served as president and chief technology officer for Harbor Fluid Products, from 2002 to 2011.
Brosius earned a BS in mechanical engineering from Stanford University and completed graduate studies in finance at the University of California, Berkeley. He also holds an honorary degree in engineering management from California Polytechnic University at San Luis Obispo.
Mark Brosius
Chief Manufacturing & Supply Chain Officer
Intuitive Surgical


Peter Bennett is the Senior Vice President of Global Supply Chain for Cardinal Health. In this role, he is accountable for International Logistics, Global Trade, Global Distribution Operations, Global planning, and key customer collaborative planning, forecasting and replenishment (CPFR) teams. He supports Cardinal Health’s Global medical product brands, national brands, Presource, and AeroMed. Bennett earned a bachelor’s degree from the United States Military Academy at West Point, and a master of science from Central Michigan University. He is a member of the Association of Supply Chain Management (ASCM) and currently services as a Co-Chair of Healthcare Industry Distributor Association (HIDA) supply chain visibility council and is on the supplier advisory council of the Healthcare Industry Resilience Collaborative (HIRC).
Peter Bennett
Senior VP Global Supply Chain
Cardinal Health


Eamonn Nestor
Senior Vice President, Orthopaedic Operations
Smith+Nephew

Terry Walsh serves as the Global Vice President of Operations at Beckman Coulter, a Danaher Company, where he is responsible for the comprehensive oversight of Manufacturing, Supply Chain Planning, Strategic Sourcing, Distribution & Logistics, Transformation/PMO, Enterprise Operational Risk, EHS, Operations Engineering, and the effective implementation of the Danaher Business System (DBS). His leadership directly impacts all businesses and channels across the extensive global network.
A staunch advocate for Danaher’s core value, “The Best Team Wins,” Terry believes in the power of diverse, inclusive, and collaborative teams to drive innovation, solve complex challenges, and ensure business success. He is deeply committed to fostering a culture of belonging, empowering associates, and achieving superior results through collective effort and data-driven decisions. This commitment extends to his sponsorship of the Operations Talent program across Danaher’s Diagnostics platform, championing continuous learning and development.
With over 30 years of distinguished experience, Terry has a proven track record in Supply Chain, Manufacturing, Organizational Development, and Global Operations leadership. Before joining Beckman Coulter in 2024, he successfully led global operations at SI Group and held significant leadership positions at Wilsonart, Henkel, Novartis, Merck, and Johnson & Johnson. His career spans both public and private equity environments, providing a broad strategic perspective. Terry holds a Bachelor of Science in Mechanical Engineering from Rutgers University and a Master of Business Administration in International Finance from Rider University.
Terry Walsh
SVP Global Operations
Beckman Coulter


Rosaleen Burke, Global Quality and Regulatory, Boston Scientific, is responsible for leading all aspects of the quality and regulatory strategy for the Boston Scientific business and broad portfolio. Roz joined Boston Scientific in 1997 and has led Quality and Regulatory teams across several regions, business units, sites and quality functions in roles of increasing responsibility and scope. Roz began her current position in 2017.
She holds a B.Sc. in Microbiology and Biochemistry and a Master’s degree in Biotechnology from the University of Galway, as well as an MBA from Oxford Brookes University. Outside of her professional pursuits, she enjoys horse riding, golf, reading, and playing Scrabble.
Rosaleen Burke
SVP, Global Quality & Regulatory
Boston Scientific


Dina is currently the Vice President of Regulatory Affairs for Terumo Medical Corporation where she oversees both US and global Regulatory Affairs. Prior to joining Terumo in 2008, Dina spent 17 years at the FDA as a reviewer and later a branch chief in the Division of Cardiovascular Devices. She also spent some time as a Sr. RA consultant prior to joining Terumo.
Dina holds a BS in Biomedical Engineering from The Catholic University of America and a Master of Business Administration from the University of Maryland.
Dina Justice
Vice President, Regulatory Affairs
Terumo Medical Corporation


Mizanu Kebede is the Chief Quality & Regulatory Officer at Smith & Nephew and serves on the company’s Executive Leadership Team. In this role, he is responsible for leading all aspects of quality and regulatory strategy development and implementation. He is also responsible for engaging directly with global regulatory agencies on all regulatory matters impacting the company’s diverse portfolio.
With over 25 years of leadership experience across the Medical Device, Pharmaceutical, and Healthcare industries, Mizanu has held senior roles spanning R&D, Operations, Commercial, and Quality & Regulatory Affairs. Prior to joining Smith & Nephew, he held executive positions at several leading healthcare companies, including Avanos Medical, Thermo Fisher Life Technologies, STERIS Corporation, and Johnson & Johnson.
Mizanu holds a Bachelor of Science in Medical Technology and a Master of Science in Microbiology and Immunology. He is a certified Six Sigma Black Belt in Process Excellence and has authored numerous publications in scientific journals. He also holds multiple patents in the medical device space.
In addition to his professional accomplishments, Mizanu is committed to community service. He serves as an Advisory Board Member for United Way of North Fulton and is actively involved with the United Way of Greater Atlanta.
Mizanu Kebede
Chief Quality & Regulatory Officer
Smith & Nephew

Steve C de Baca is Executive Vice President and Chief Patient Safety & Quality Officer of Royal Philips. He brings more than 35 years of quality and regulatory affairs (QRA) experience in the medical technology industry.
Prior to joining Philips in February 2023, Steve served as the EVP of QRA for Cardinal Health, supporting the $185B enterprise and was a member of the Operating Committee.
Prior to this, Steve served as VP of QRA for Orthopedics and Americas for Zimmer Biomet, where he was responsible for supporting multiple business segments as well as the Americas region for international regulatory and compliance. Earlier in his career, he served as the SVP of Quality, Regulatory & Clinical Affairs for the Danaher Diagnostic platform, which includes the four separate operating companies of Beckman Coulter Diagnostics, Leica Biosystems, Radiometer & Cepheid. Prior to that, Steve was with Boston Scientific for nearly nine years in progressively senior strategic roles, the last of which was VP of Quality, for the Cardio, Rhythm, and Vascular (CRV) and the Neuromodulation businesses.
Additionally, Steve served as an industry board member and instructor for six years for the Regulatory Affairs master’s degree program at St. Cloud State University. He earned a bachelor’s degree in engineering and industrial technology and an MBA, both at California State University Long Beach.
He lives in Minnesota and has been married to his wife Katherine for over 26 years. They are proud of their son Carson who is a graduate from Northeastern University in Boston and currently living in Chicago. Steve is an avid runner and sports enthusiast.
Steve C de Baca
Chief Patient Safety & Quality Officer
Philips


David Kunz was appointed Senior Vice President, Global Quality and Regulatory Affairs in May 2017. He is responsible for providing strategic and operational direction to achieve Quality and Regulatory Excellence across the organization and he chairs the Company’s Quality Excellence Steering Committee. Mr. Kunz joined the Company (then Zimmer) in 2011 as Vice President, Regulatory Affairs and Quality Assurance for the Spine division. He was appointed Senior Vice President, Global Quality, Clinical and Regulatory Affairs in April 2016. Prior to joining Zimmer, he served as Vice President, Quality Assurance of Ecolab, where he led a supply chain network including nine chemical plants and two equipment plants. Previously, he spent 13 years at Guidant/Boston Scientific Corporation in a variety of leadership positions, most recently as the Director of Supplier Quality Assurance for the CRV Division. He began his career at the Naval Nuclear Propulsion Program Headquarters as a mechanical engineer. Mr. Kunz holds a Bachelor of Science and an MBA from the University of Minnesota. He is a certified Nuclear Engineer and previously was a certified Licensed Professional Mechanical Engineer.
David Kunz
Senior Vice President, Global Quality & Regulatory Affairs
Zimmer Biomet

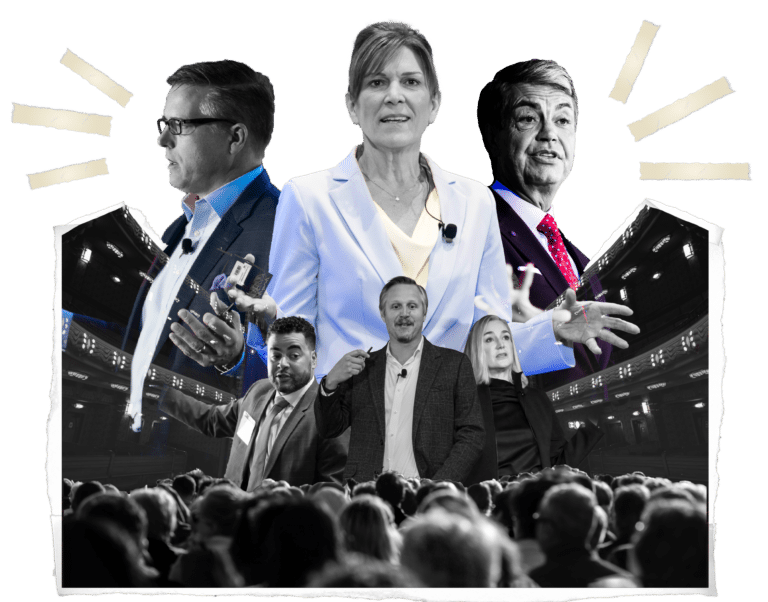
The Medical Device Manufacturing Executive Summit 2026 is designed to ensure every attendee walks away with real & measurable strategies to bring back to their organizations.
Director of Quality, Contracted Services, Nissha Medical Technologies
Plant Manager – Alajuela Costa Rica, Philips
Vice President of Innovation, MicroAire Surgical Instruments
Sr. Manager Quality Relations, Olympus
Director of Operations, Nectero Medical
Sr. Director, THV Global Operations, Jenavalve



















